Testing Sites in Austin
COVID VACCINE
Covid-19 Vaccination sites in Austin provided by the City of Austin:
Go to the link below to pre-register for the vaccine from Austin Public Health, then you can search for distribution sites and make an appt OR if none are available, come back to the site later to search again. FYI: NextDoor bulletin board sometimes posts announcements of vaccine availability.
Click here: http://austintexas.gov/covid19-vaccines
Tell us about your experience finding the COVID vaccine. Are you on a waiting list? What were you told would happen when the vaccine becomes available? Have you gotten the vaccine? Where did you get it? Did you have to pay anything? What could you share that would help other Austinites get vaccinated? You don’t have to give your name. We’d just like to be a resource for other Austinites, especially LGBTQ+ seniors. You can post to our FACEBOOK PAGE at:
https://www.facebook.com/algbtcoa
Austin – Travis County
COVID-19 Dashboard Public Datasets
https://atc-covid19data-austin.hub.arcgis.com/
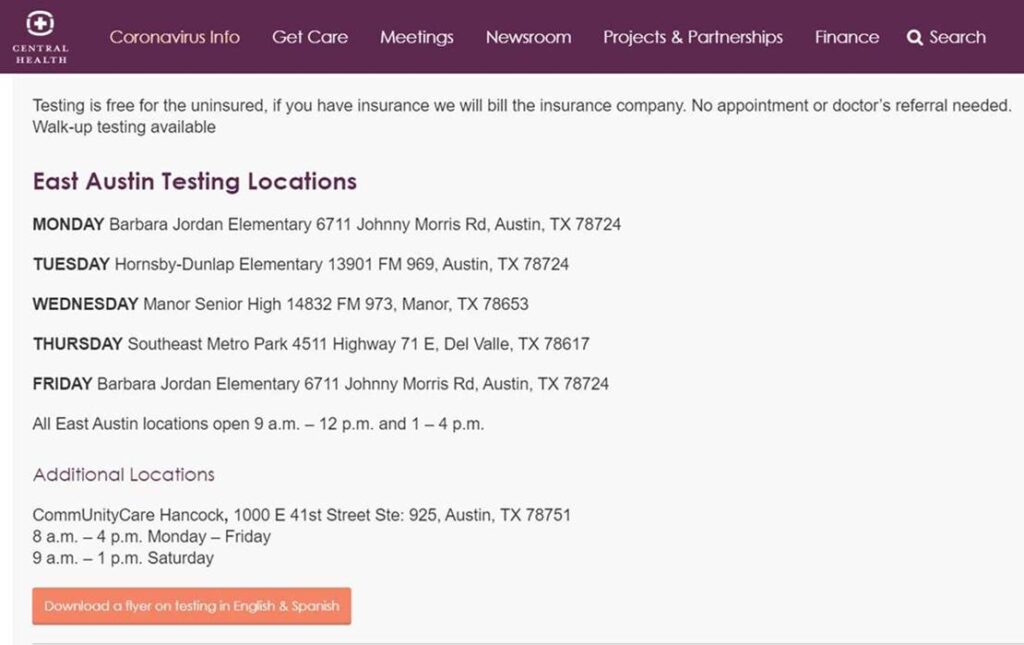
Coronavirus Testing Sites in Austin
click on the image below
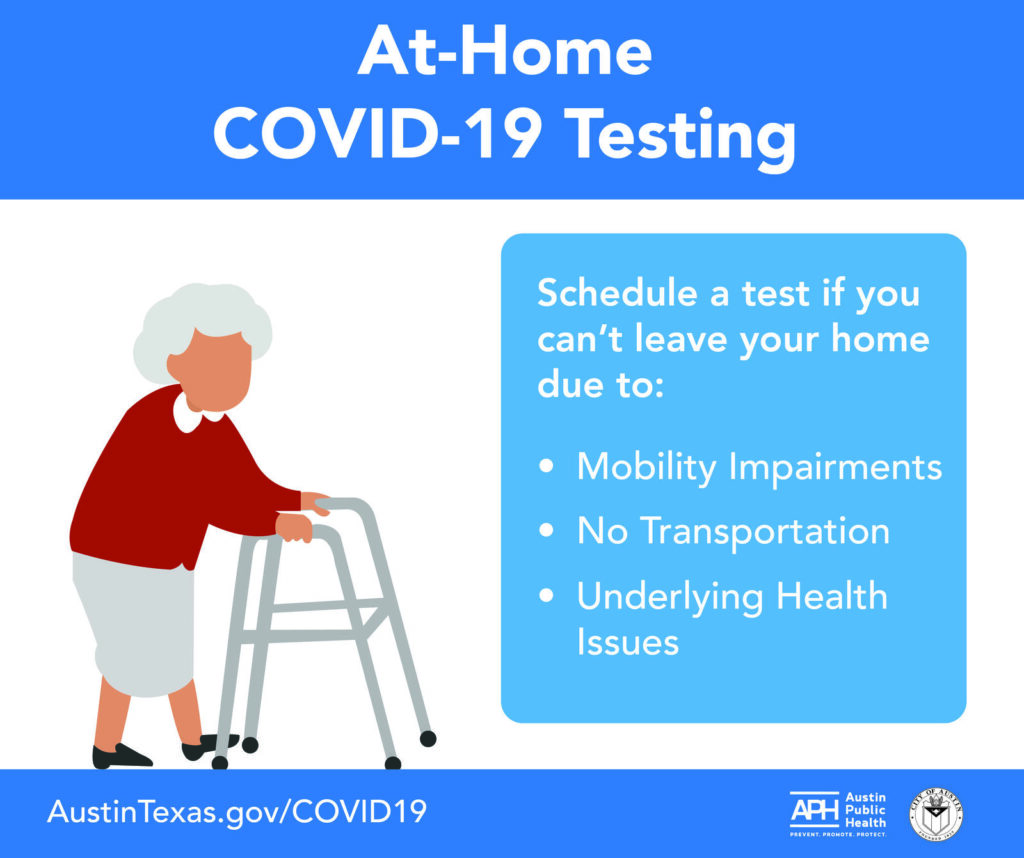
City of Austin announces at-home testing; they’ll come to you; call them.
At-Home Testing. At-home #COVID19 testing is available! ? If you can’t leave your home due to mobility impairments, are considered to be at high-risk of developing severe symptoms with or don’t have transportation, you can schedule a test. We come to you! Call (512) 972-5560.
¡La prueba del #COVID19 en casa ya está disponible! ? Si no puede salir de su casa debido a problemas de movilidad, problemas de salud subyacentes o no tiene transportación, puede programar una prueba. ¡Nosotros venimos a ti! Llame al (512) 972-5560.
The Administration for Community Living
has sent out guidelines that provide guidance for respite care agencies and other respite providers who are planning to resume services. It includes strategies to family caregivers and providers on providing and receiving respite care as safely as possible during COVID-19.
Here’s the link: https://archrespite.org/national-respite-guidelines-for-covid-19.
Texas Provides Guidance for Nursing Facilities : Current Guidance Addresses Infection Control Recommendations
AUSTIN – Texas Health and Human Services is releasing current guidance to nursing facilities across the state on how to respond in the event of a presumptive or confirmed case of COVID-19.
This current guidance – COVID-19 Response for Long-term Care Facilities – includes an overview of information provided to nursing facilities and highlights requirements and recommendations to protect resident health and safety. The guidance applies when a facility becomes aware of the infection of a resident, staff member, or visitor.
“We hope this information will help the providers we regulate respond as effectively as they can to COVID-19, as we fully understand they are confronting an unprecedented situation,” said David Kostroun, Deputy Executive Commissioner of the Texas HHS Regulatory Services division. “This detailed guidance spells out the immediate, short-term and longer-term steps a facility should take to help contain the spread of the virus, including protocols for infection control, isolation of residents, and the use of personal protective equipment. This plan reflects our ongoing commitment to give providers the information they need to protect the vulnerable people they serve.”
A result of interagency collaboration among HHS, the Department of State Health Services and the Texas Division of Emergency Management, this current guidance is posted on the Texas HHS website here:
The guidance is a living document that will be updated frequently as new guidance is provided, so facilities are urged to check this page regularly.
Long-term care facilities are reminded they must notify HHS and their local health authorities of any presumptive or confirmed case of COVID-19 for a resident or staff. An HHS survey team then actively investigates to assess the facility’s compliance with all relevant health and safety rules, including those requiring it to follow proper infection control practices.
All long-term care facilities where COVID-19 has been detected also must follow all CDC and Texas DSHS guidelines for infection control, including appropriate use of isolation and personal protective equipment for staff and residents, to protect health and safety. Guidance for facilities also includes working closely with local health officials.
More information is available at hhs.texas.gov. Texas residents can dial 2-1-1 to learn about programs and services.
______